Originally published in Pharma Manufacturing Magazine
Pharmaceutical manufacturers participate in a strictly regulated, high-stakes industry. Strictly regulated for good reason. The process and the product impact the lives of millions of people around the world. The production process must exist in a controlled environment and the product must be transported within specific environmental conditions to maintain product safety and efficacy.
In this industry, profits can be achieved only if the company is driven by a passion for healing and helping people, rather than for only maintaining a profitable manufacturing process. Process and product safety — from research and development to therapy delivery — must always be in good balance with costs.
Time to market is more important than ever so therapies can reach new markets, compete with generics, and get the most out of products before their patents expire. Delivering the highest quality product while producing it efficiently requires finely engineered tools and software as well as insightful methods of gathering and using information. The key is creating a connected enterprise by harnessing the power of an integrated control and information system. The industry is still learning.
Recently, hard-fought battles have been won to create life-saving vaccines only to have them be destroyed by falling out of compliance on their way to the patients in need. This was a life-threatening failure in the proper chain of custody, or supply chain.
Manufacturers are evolving to avoid these failures by moving toward a strong philosophy supported by better use of measurement devices and environmental monitoring systems — among other innovative solutions — so that speed to market can be realized.
PHARMA 4.0 AND THE PHARMA SUPPLY CHAIN
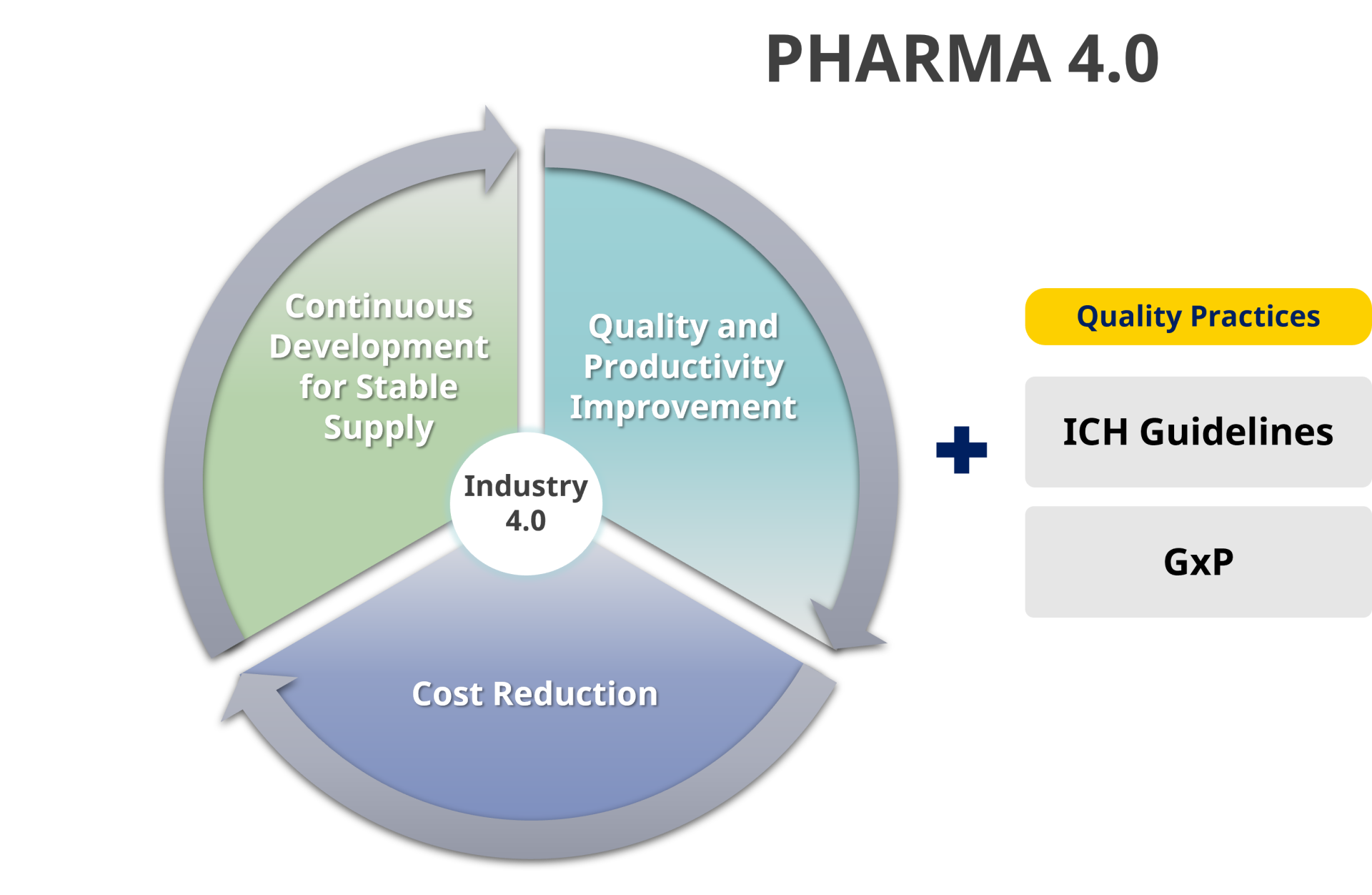
As the pharmaceutical industry continues to design and document medical therapies’ chains of custody, they seek guideposts and tools to help streamline the processes and reporting. They recognize that combining measurement and control with a simple data historian will not deliver the required results.
The International Society for Pharmaceutical Engineering (ISPE) suggests a path forward. Pharma 4.0 (Exhibit 1) is a holistic operating model that leverages information technology advances to drive quality and productivity improvements, reduce costs, and develop stable supply chains.
Pharma 4.0 — based around the Indus- try 4.0 elements of people, process, and technology — adds the strong influencer of culture to the mix. By doing so, the philosophy seeks to embed the requirements of life safety into the daily processes and attempts to simplify the transformations and embed proper decision-making into the processes.
Pharma 4.0 is vital to transforming the supply chain if the industry and their partners are to meet future demands. In this new pharmaceutical manufacturing era, the industry seeks increased efficiencies through process visibility, faster decision-making, and real-time system optimization so that they can radically reorient their business process for safe, reliable, and profitable operations. Techniques used in the past must evolve as they will not enable the industry to maintain pace with the new therapies that are required.
Achieving Pharma 4.0 requires a future-focused vision encompassing a thorough understanding of pharmaceutical quality systems, measurement and control solutions, industry regulations, and practical IT implementation skills so digital technologies are adapted to
empower the people, process, and technology along with the culture — making pharmaceutical manufacturing seamless.
Practices in IT are critical because they leverage the internet of things (IoT) and artificial intelligence (AI) to use data and information in transformative ways. This includes effective analysis of the massive data collected from manufacturing equipment, sensors, and processes as well as real-time monitoring for deviations or changes.
By moving to Pharma 4.0, manufacturers will realize improved flexibility, timely response in the face of changes, less waste, and greater visibility to materials in the process. Every pharmaceutical manufacturer has slowly started to spend money on equipment that is fit for the future — for them, that means digitalization above all.
If it sounds exciting, it is. If it also sounds daunting, it can be.
Evaluate organizational readiness
 The list of projects required to move an organization to Pharma 4.0 conditions might seem overwhelming, but it can be tamed by starting with a readiness assessment. Some pharmaceutical manufacturing partners, such as Yokogawa, use the Smart Industry Readiness Index (S.I.R.I.) created by the Singapore Economic Development Board in 2017 in partnership with consulting firms and industrial partners. Yokogawa has over 35 Certified S.I.R.I. Assessors (CSA) to per- form the readiness assessments.
The list of projects required to move an organization to Pharma 4.0 conditions might seem overwhelming, but it can be tamed by starting with a readiness assessment. Some pharmaceutical manufacturing partners, such as Yokogawa, use the Smart Industry Readiness Index (S.I.R.I.) created by the Singapore Economic Development Board in 2017 in partnership with consulting firms and industrial partners. Yokogawa has over 35 Certified S.I.R.I. Assessors (CSA) to per- form the readiness assessments.
Because “you can’t improve what you don’t measure,” Yokogawa’s CSAs assess an organization’s readiness for digital transformation. By understanding critical components of a future-ready manufacturing facility, an organization can be guided by the S.I.R.I. frameworks and tools to start, scale, and sustain their transformation.
At Yokogawa, we envision a future in which pharmaceutical manufacturing processes — from sales and production planning to actual manufacturing operations — will be coordinated and autonomous. Monitoring, controlling, sensing, visualization, and prediction technologies can make a significant contribution to the autonomy of manufacturing operations. In addition, delivery to patients can be autonomous, and the optimal production volume according to demand will be fed back to the field, contributing to the reduction of therapy loss (disposal of expired therapies) in cooperation with medical care.

Moving to this future requires real-time and historical data. The S.I.R.I. assessment will identify gaps in data gathering and analysis abilities so the pharmaceutical manufacturers can maintain product quality — from the storage and use of raw materials, to the intermediate products that are manufactured, till the final pharmaceutical product that is formulated and dispatched to the final user destination.
All these therapy stages are directly affected by the environments in which they are handled and have an impact on their quality across the entire supply chain. From raw materials to final therapies, the supply chain and the environment must be monitored. Many of the gaps in data can be addressed by including a well-designed environmental monitoring system (EMS).
EMS AS PART OF THE SUSTAINABLE SUPPLY CHAIN JOURNEY
Digital maturity supports the supply chain and requires measurement of conditions throughout the process as well as alerts to changes and proper actions. Central to the digital supply chain solution is the EMS, which collects, maintains, and distributes data for
monitoring the environment in the pharmaceutical manufacturing, quality control, and storage areas (Exhibit 2).
For a sustainable supply chain, the monitoring system must be embedded into the production and distribution processes. In fact, the ideal EMS must be able to store data from areas such as small-scale monitoring of freezers, refrigerators, and chambers; large-scale data monitoring of manufacturing processes and clean rooms; and temperature and humidity monitoring in warehouses and storage facilities.
The production and storage data must be available digitally (Exhibit 3) to the people and organizations that need it — making it easier and less time-consuming to ensure the supply chain quality while maintaining and creating reports.
To perform each of these process activities in the proper conditions, the tools and techniques must be fit-for-purpose — designed with the pharma manufacturers in mind so they can meet the compliance regulations and prove compliance. An EMS should support many characteristics specifically designed for the pharmaceutical industry in all areas that support the supply chain (Exhibit 4).

Environmental monitoring plays a critical role in ensuring the safety of patients and the efficacy of the therapies and biologics by preventing their contamination with microbes — not just on the manufacturing side, but also on the R&D side and the delivery side of the therapies.
In the R&D testing environment the cleanliness, the temperature, the pressure, the humidity, and more must be continuously monitored using an EMS that is in line with ALCOA+ requirements to guarantee the safe production of the therapies in the R&D laboratory.
Logistics, inventory management, and warehouse management play key roles in manufacturing the right product at the right quantity so that the final therapy is made available for the right people at the right time. The quality of the raw materials used for therapy manufacture (APIs produced or the final therapies produced) must be stored, transported, and maintained in the right environment. Non-compliance in the right environment will have an impact on product quality and will affect safety, productivity, profitability, and could lead to losing the license to operate.
CREATING PARTNERSHIPS
Generally speaking, what is good for a community is good for a pharma company. More specifically, if a pharma company aims to design therapies to help people, produces them in a proven and reliable process, and distributes them safely to the people that need them, that company will succeed and grow. And a supply chain that is sustainable from R&D to delivery will support that company.
In moving toward those goals, much depends on the vendor and supplier partnerships developed with the pharmaceutical companies. The partners helping develop the supply-chain processes and tools must be as dedicated and passionate as an organization that puts its name on therapies.
Industrias
-
Farmacéutica
La industria farmacéutica enfrenta actualmente el importante reto de aprovechar al máximo las oportunidades que se presentan en los grandes mercados emergentes. Ahora, más que nunca, las compañías farmacéuticas necesitan introducir técnicas de fabricación ajustada que mejoren la rentabilidad. Siendo uno de los principales proveedores de automatización en el mundo, Yokogawa asume el compromiso de suministrar las mejores soluciones posibles para sus mejores prácticas de fabricación.
Productos y Soluciones Relacionadas
-
OpreX Environmental Monitoring System
The OpreX™ Environmental Monitoring System collects, measures, and stores temperature and humidity as well as other management data for comprehensive oversight of the pharmaceutical manufacturing, quality control, and storage areas.
-
OpreX Laboratory Information Management System
OpreX Laboratory Information Management System(hereinafter "OpreX LIMS") is a Laboratory Information Management System (LIMS) package, which supports quality management operations. Furthermore, it standardizes quality management operations to reduce costs and improve the service level.
-
Digital Transformation
- Yokogawa's wide process manufacturing knowledge/expertise
- Digital transformation solutions for a better future for our customers
Have Questions?
Contact a Yokogawa Expert to learn how we can help you solve your challenges.