What are organoids?
Stem cells have been used in research for many years. However, game-changing was the possibility to grow stem cells in three dimensions (3D) to build miniature organs (organoids) that can mimic many aspects of parent tissue in a dish. Organoids recapitulate the genetics, cellular organization, and to a certain extent the functionality of the corresponding parent organ.
Organoid models are available for researchers for diverse organs, cell types, cell environments, or disease states and can be patient-derived from human donors, too.
What are organoids used for?
During the last years, 3D organoid models have become more and more important. They are usable for many researchers and applications. The application range covers from simple tumor models to more complex co-culture systems. Organoids are now widely used as a model for drug discovery but also for drug toxicity studies. Thus, organoids may help reduce the number of animal studies in the future.
Organoids are superior to flat 2D cell cultures in their physiological relevance, increased cellular complexity, and yet can be used for screening.
Screening in organoids
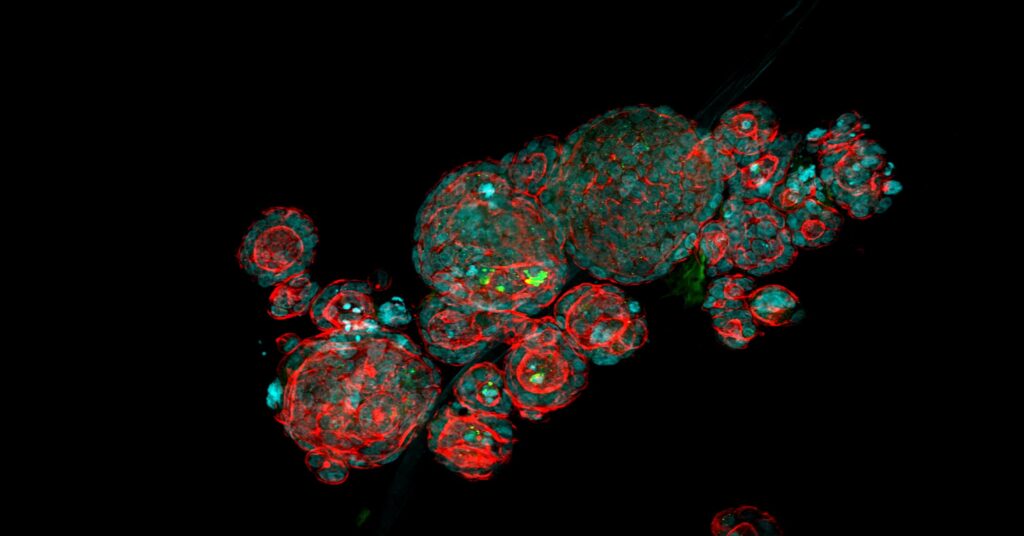
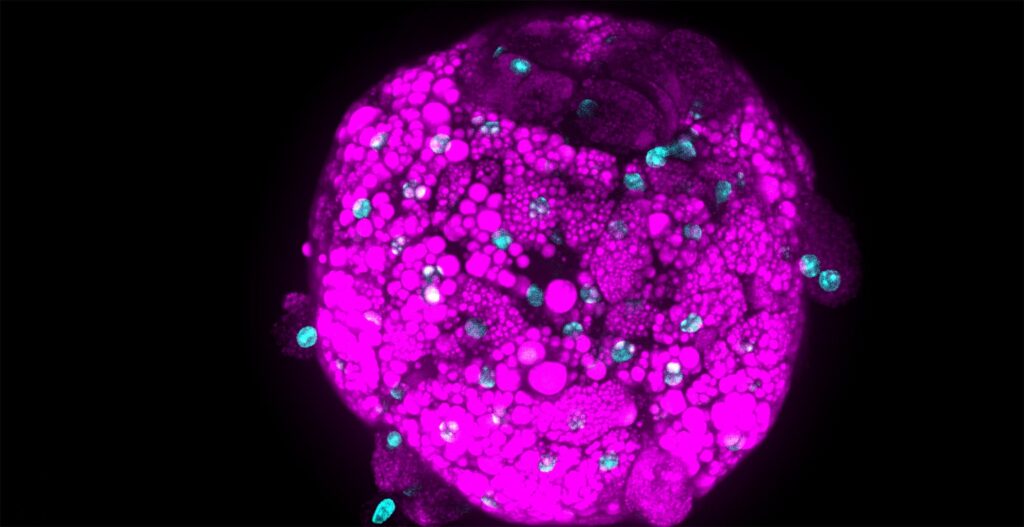
The research team of Prof. Prisca Liberali at the Friedrich Miescher Institute for Biomedical Research (FMI) in Basel explored the potential of organoids for drug screening in a unique image-based screening approach assaying an annotated library of compounds to elucidate tissue regenerative mechanisms in organoids. Her group used intestinal organoid cultures to understand what drives their ability to regenerate and self-organize. So far, the underlying mechanisms controlling organoid self-organization are poorly understood. To understand how self-organization is maintained and regulated Prisca’s team mapped thousands of functional genetic interactions.
[vc_cta h2=”Webinar: High content screening reveals the phenotypic landscape of intestinal organoid regeneration” h4=”March 23, 2021 5 PM CET” add_button=”bottom” btn_title=”Register now!” btn_color=”turquoise” btn_link=”url:https%3A%2F%2Fwww.drugtargetreview.com%2Fwebinar%2F80805%2Fhigh-content-screening-reveals-the-phenotypic-landscape-of-intestinal-organoid-regeneration%2F%3Ftarget%3Dregister%26campaign_source%3DEuropeanheadquarters%23register|title:Register%20now|target:_blank” css=”.vc_custom_1616164895740{background-color: #00a04c !important;}”]Ilya Lukonin, Ph.D. at FMI Basel will explore the application of complex and biologically relevant model systems for drug screening, such as small intestinal organoids.
[/vc_cta]
How can you set up such a screen?
First of all, one needs to have a powerful infrastructure available. Your technical stack includes a high-end microscope, computing, and IT infrastructure, to set up such a demanding screen.
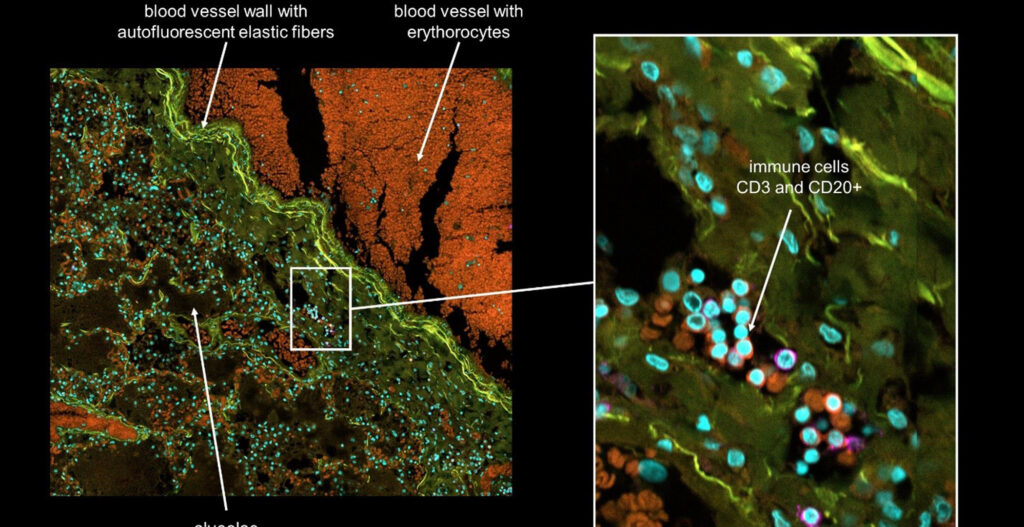
Then, one needs to define a read-out on the cellular or organoid level. That means if you expect tiny structural changes on the cellular level one may ask for a confocal microscope with a classic spinning disc unit as equipment. For instance, you could use the confocal scanning units (CSU) from Yokogawa.
Of course, the following question will need to address the imaging speed and screen size. Both questions need to be answered together as they need to be balanced. In other words, a smaller sample number allows for a system that may image with one camera. I would recommend a system like Yokogawa’s CQ1 Confocal Imaging Cytometer.
The Yokogawa CQ1 Confocal Imaging Cytometer was developed with a view to Yokogawa’s extensive experience with high content systems. Such as the CellVoyager 7000 or 8000 and the numerous CSU optical units, which have also been used in competitor systems for many years.
Prisca’s team however went for a huge sample number. They established an image-based phenotypic screening platform, profiling over 450,000 organoids treated with a library of compounds. That means the speed of a suitable confocal microscope mattered a lot. The CellVoyager from Yokogawa can deliver the needed imaging speed and image quality, because it may image with multiple cameras in parallel. The new Yokogawa CV8000 system enables simultaneous 4-color imaging of live and fixed cells.
A phenotypic organoid fingerprint
In their study, authors created unique “phenotypic fingerprints” to describe every compound screened at the single organoid level.
With this approach, Prisca’s team identified and described the role of 230 genes that are involved in organoid development.
To cut a long story short, the researchers identified a compound that improves the regeneration of the intestine in vivo thereby paving the way for novel therapies. Furthermore: the screening approach can be employed for different scientific questions and new projects.
The article „Phenotypic landscape of intestinal organoid Regeneration“ was recently published in Nature (Lukonin et al, Nature 2020) and gained a lot of attention in the community. An amazing organoid image even made it on the Nature Cover. Yokogawa would like to congratulate the authors (Prisca Liberali and Ilya Lukonin) on this big success.
https://twitter.com/nature/status/1313878976637665281″ el_aspect=”43
Do you want to know more about our CQ1? Just approach us via the contact form or send an e-mail to lifeinnovation@de.yokogawa.com.
For more information about Prof. Prisca Liberali’s research, please visit liberalilab.org.