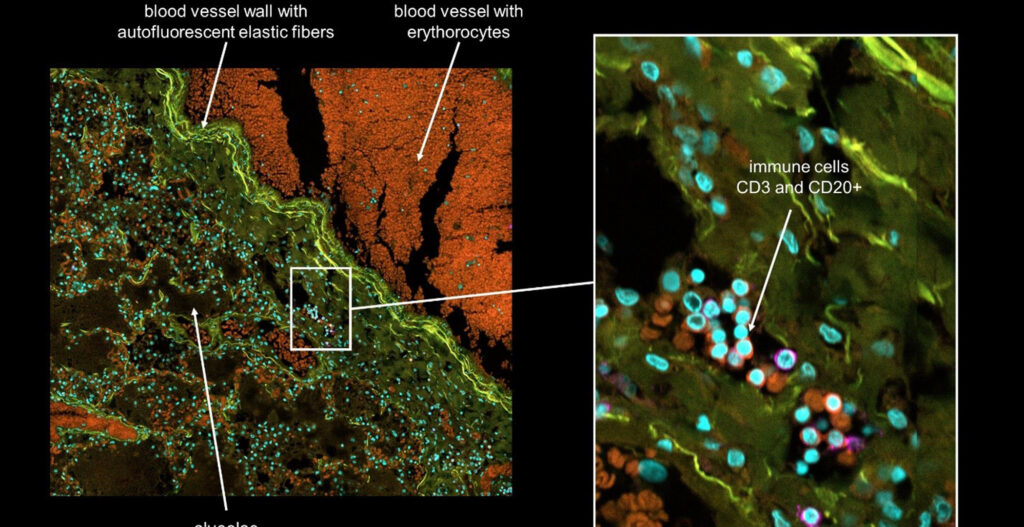
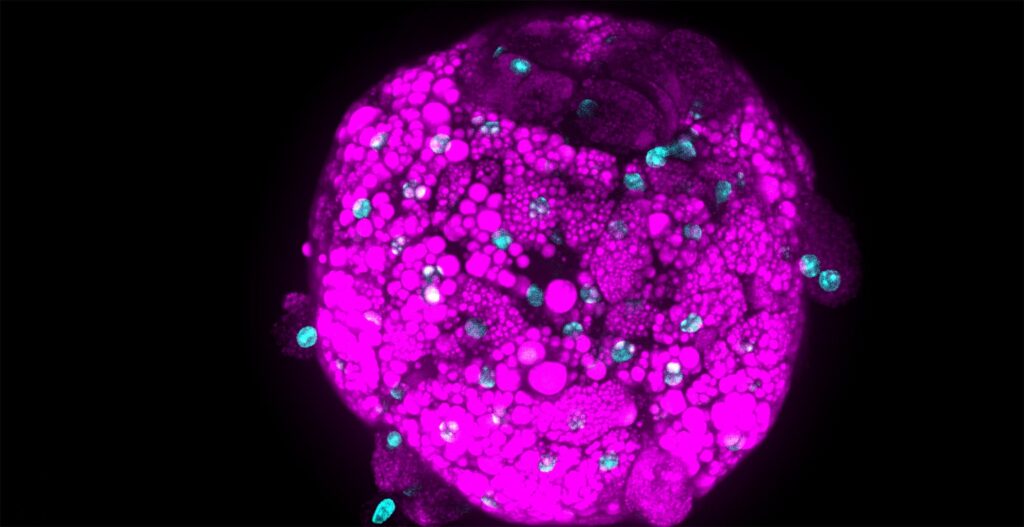
Today, we are delighted to have Sven Fengler, Ph.D., a talented biologist with expertise in microscopy and quantitative imaging. Let’s delve into his fascinating journey and groundbreaking work in which he provides the necessary technology to find a cure for neurodegenerative diseases like Parkinson and Alzheimer.
LI Blog: Could you tell us about your academic journey and how you developed a keen interest in microscopy and quantitative imaging?

Technologies (LAT) lab of the Core Research Facilities and Services (CRFS) in Bonn, Germany
Sven Fengler: Absolutely! I am a biologist by training. My academic journey began with my undergraduate studies at the University of Cologne. Conducting quantitative FRET experiments by acceptor photobleaching allowed me to explore protein-protein interactions and caspase activity, particularly in the context of the NF-κB-inflammation signaling cascade, which involved a myriad of proteins. This experience ignited my passion for using advanced imaging techniques to study intrinsic biological processes.
LI Blog: How did your Ph.D. research contribute to your expertise in microscopy?
Sven Fengler: My time at the Max Planck Institute in Dortmund, working on my Ph.D., was transformative. Particularly combining FRET-FLIM assays with automated microscopy, allowed me to explore protein phosphorylation events inside cells with spatial resolution in a high-content format. I used this technique to investigate the phosphorylation status of the EGF-receptor and its regulation by phosphatases during receptor signaling and recycling events. I developed reverse transfection cell arrays that allowed me to express 384 different proteins from cDNA libraries on a miniaturized format with a size of a microscope slide. Part of my work was to automate the cell array production for my PhD project.
LI Blog: How did working with physicists influence your work?
Sven Fengler: It was inspiring to work together with physicists in the team because it allowed me to gain a deep understanding of the technical aspects in laser optics and quantitative image analysis. In particular, working in a team of experts to develop an automated microscopy setup to image transfection cell arrays with FRET-FLIM optics was groundbreaking for me.
LI Blog: As an early PostDoc, you transitioned to DZNE Bonn. Could you tell us about your work there?
At the DZNE Bonn, I found the perfect match for my skills and interests. Therefore, I joined the team of Laboratory Automation Technologies (LAT) within the Core Research Facilities and Services (CRFS) in 2017. My work focuses on the assay development of advanced human iPSC-derived cell models for complex 3D systems and preclinical drug screenings. The dynamic research environment at DZNE Bonn has allowed me to grow professionally and personally.
Research Focus
LI Blog: What is your main research focus?
Sven Fengler: Recently, we have established a novel blood-brain barrier (BBB) system, which I have also presented at the CV8000 User Group Meeting in 2022. The constructed blood-brain barrier endothelium of the system derives from human iPS cells. In the past years, we developed a 3D microvessel system, like a small blood vessel in vitro. The aim was to establish a screenable system that mimics the complex architecture of the human BBB as close as possible to provide a technology to screen for BBB-penetrating substances early – in the phase of preclinical drug discovery.

The Importance of the Blood-Brain Barrier
LI Blog: What is the blood-brain barrier (BBB), and why is it important to study the BBB?
Sven Fengler: This specialized endothelial structure, present in all of us, plays a crucial role in maintaining healthy brain function and preserving the brain’s delicate internal environment. This selective permeable barrier allows essential nutrients and molecules to enter the brain while keeping toxic substances at bay. The key players in this defense are efflux transporters, which actively pump out harmful substances back to the blood cycle. By doing so, these transporters safeguard the brain’s delicate microenvironment, ensuring it remains free from detrimental agents that could disrupt neurological homeostasis. However, when you think about designing a drug against Parkinson or Alzheimer diseases for example, you need to overcome this barrier and “trick” these transporters, the endothelium per se. Overcoming this challenge is crucial for the success of potential treatments in general. The high failure rate of drug candidates in clinical trials can often be attributed to their inability to penetrate the BBB effectively.
Recent iPS cell technology has the potential to mimic the complex architecture of the human BBB much closer than it was possible a couple of years ago. Such systems could help us to test and finally better predict a BBB-penetration of a new drug.
The Drug Discovery Process for Neurodegenerative Diseases
LI Blog: Could you explain to us the conventional drug discovery process for neurodegenerative diseases?
Sven Fengler: During lead optimization, researchers employ a variety of experiments to predict the drug’s ability to cross the BBB. Often, these assays use artificial membranes or Transwells, which may not accurately replicate the physiological conditions of the human BBB. For example, Transwells experiments typically comprised of non-brain tissue cell lines, might not exactly mimic the relevant properties of the very complex BBB system present in the human brain.
LI Blog: So, there are limits in preclinical testings?
Sven Fengler: Exactly. When potential drug candidates fulfill a consortium of properties measured in various in vitro assays, researchers assess whether a drug can penetrate the mouse brain to generate relevant in vivo data. However, optimizing lead-compounds based on mouse BBB permeability might not yield results that accurately represent the human BBB’s behavior. Consequently, many drug candidates may fail later during clinical phases due to their inability to cross the human BBB effectively.
LI Blog: Why could iPS cells overcome these hurdles?
Sven Fengler: We are hopeful that iPSCs can offer a more relevant and human-specific approach to address BBB permeability concerns. iPSCs can be generated from human tissues and have properties that mimic human BBB physiology better than traditional approaches. By using iPSCs to create endothelial cells that closely resemble the physiological brain endothelium, scientists can establish a more accurate model of the BBB. iPSC-based endothelium offers a regenerative system that can be grown for extended periods while maintaining characteristics closer to primary human cells. This advantage is particularly relevant for testing substances over a long experimental time. By measuring the Trans-Endothelial Electrical Resistance (TEER) of these cells, researchers can assess the integrity of the endothelium, gauging whether it forms a robust and tight barrier, akin to the human BBB.
3D human iPSC-derived Blood-Brain Barrier System – Technical Details
LI Blog: What is the technical background of your 3D human iPSC-derived blood-brain barrier system on a chip?
Sven Fengler: In our endeavors to mimic the physiological state of the BBB, we have characterized tight-junction proteins expressed by endothelial cells. These proteins play a pivotal role in the “passive” barrier function of the BBB. By measuring the TEER of our 3D endothelial vessel, the barrier property can be assessed quantitatively. To that end, our generated endothelium in culture shows physiological relevant barrier properties by reaching TEER values that resembles those measured in rodent brain vessels. We could experimentally show that our 3D system mimics the complexity of BBB architecture, including polarized efflux transporter localization. Finally, we simulate a medium flow that closely resembles the blood flow in small blood vessels.
LI Blog: Do you use special plates for imaging?
Sven Fengler: By seeding our IPSC-derived endothelial cells into the microfluidics chip (OrganPlate® 3-lane 40) from Mimetas, remarkably, the endothelium self-organized into a vessel within two days. The grown vessel exhibit wound-healing properties by keeping its mono-layer structure that requires perfusion for optimal functionality. Using Mimetas plates, the medium perfusion is applied by a simple gravity flow simulating the blood flow inside each vessel. Each plate can contain up to 40 microvessels, providing sample testing capacity. Additionally, the system is compatible with imaging, allowing us to observe and analyze the microvessels and their interactions with other brain cells.
LI Blog: How was the imaging further performed?
Sven Fengler: For the microvessel characterization as well as for the high-content analysis in our permeability assays, we’ve used the Yokogawa Cell Voyager CV8000. This state-of-the-art system allows us to image the microvessels fully automated and obtain fast, stitched 3D stacks with different fluorescent channels. The speed and efficiency of this system have enabled us to generate a wealth of data. Due to the integrated incubator system, live-cell imaging conditions are excellent. In this context, we have used the CV8000 to visualize the BBB penetration of different fluorescent tracer molecules to test microvessel integrity and to screen for toxic substances.

LI Blog: Could your model potentially enhance the success rate in drug discovery by neglecting costs at the same time?
Sven Fengler: By simulating the physiological state in vitro, our model provides valuable insights into how novel compounds might interact with the human BBB before testing them in vivo or clinical trials. With our system, we could evaluate a wide range of drug candidates and identify those with a relevant human BBB penetration already during preclinical drug discovery phases and lead optimization. This might enable researchers to focus resources on the most promising candidates and increase the likelihood of successful clinical translation.
Ideal Users of the 3D human iPSC-derived Blood-Brain Barrier System on a Chip
LI Blog: Who would benefit the most from using your system?
Sven Fengler:
Our model’s scalability and imaging capabilities further enhance its utility, allowing us to generate a vast amount of data and create comprehensive 3D animations for analysis. Possible applications are not limited to drug discovery alone. Our model can be applied in many other research fields including immune cell migration, virus and capsid penetration or toxicology for example. A huge advantage would be less animal testing in general because our system could provide an alternative to animal models in future.
Ultimately, our vision is to leverage this technology to streamline drug development, reduce failures in late-stage clinical trials, and pave the way for more effective treatments for neurodegenerative disorders.
LI Blog: What did you investigate exactly with the system?
Sven Fengler: In particular, we have tested the BBB-penetration of a set of anti-inflammatory substances. It has been shown that inflammatory processes are involved in the progression of many neurodegenerative diseases. Therefore, we focused on the identification of BBB-penetrating anti-inflammatory compounds because this group of drugs could have a potential to cure or stop disease progression in various forms of dementia.
Outlook
LI Blog: You have already achieved a lot with the 3D blood-brain barrier system. What are your future research plans?
Sven Fengler:
I’d like to mimic a blood-brain barrier from patients with a neurodegenerative disease. For this, reprogrammed iPSCs derived from patient blood cells with a genetic disease background could be differentiated and matured to endothelial microvessels. Such an in vitro system could be used to simulate the effect of a compromised BBB due to the given type of dementia. For example, drugs could penetrate the BBB much stronger in case the BBB is leaky due to a specific disease and free drug concentrations could strongly differ from healthy to disease individuals.
Recently, we have set up our automated iPSC production robotics platform to produce iPSC-derived cells under reproducible conditions. We established protocols to freeze fully differentiated ready-to-use brain endothelial cells for our current partners in-house and for upcoming external collaborations.
LI Blog: Your research could be a lifesaver. Thank you so much for sharing your journey with us. Your work at the intersection of biology and microscopy is truly inspiring! We wish you continued success in your research at DZNE Bonn.