Manufacturers across industries understand that measuring pH in brine solutions, or saltwater brings challenges that — left unattended — lead to product waste, increased device inventory, inefficient operation, and high maintenance costs.
Challenges to pH measurements in brine are faced by many manufacturers, includes cheesemakers, cooling system designers, de-icing manufacturers, desalination systems, and most heavily those in the Chlor-alkali sector. Their energy-intensive manufacturing process includes the electrolysis of a salt solution to produce sodium hydroxide/caustic (NaOH). The by-products are hydrogen gas (H2), chlorine gas (Cl2), and hydrochloric acid (HCl).
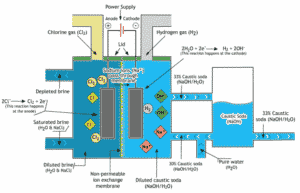
Source: https://www.eurochlor.org/about-chlor-alkali/how-are-chlorine-and-caustic-soda-made/membrane-cell-process/
The brine solution can negatively affect the production process by reducing sensor life, which potentially can go unnoticed by the manufacturer. Exposed to the brine solution, the sensor in the measurement device can become corroded, plugged up, or experience glass fractures. In addition, the sensor or the product itself can become contaminated.
The Importance of pH Measurement to Quality
For any process to be profitable, manufacturers strive for the most effective production with minimized maintenance costs. That’s why pH measurement in brine is so important. Without accurate and consistent pH measurements, chemical dosing — controlled based on the pH measurements — is not possible. If pH measurements in the brine are inaccurate, the dosage of chemicals will be incorrect.
Proper measurement of pH in brine solutions means
- Improved process efficiency
- Avoiding extra chemical costs and wasted product
- Less risk to operation
“Before updating our pH analyzers, maintenance was time-consuming and costly. With conventional technology, we encountered frequent breakdowns and a lot of staff overtime. Plus, the old sensors were difficult to use and we felt the measurement results could be improved.”
-Current Yokogawa measurement user at Nouryon Industrial Chemicals
Why and Where Challenges Arise
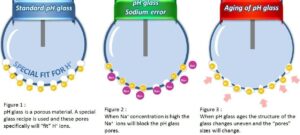
Each of these conditions shortens sensor life by causing liquid junction coating, plugging, and the poisoning of the sensor — thereby increasing maintenance and replacement costs.
Overcoming the Challenges
How can a manufacturer anticipate and address the issues while creating a more efficient and cost-effective process?
Manufacturers overcome measurement challenges today through advances in technologies and innovative techniques. This article series will investigate conditions that make pH measurement difficult while illustrating current successful solutions.
- By changing the way sensors are designed, measurement devices can successfully monitor pH in highly corrosive environments.
- When an improved type of glass is used in measurement devices, high temperatures do not negatively impact operation.
- Cation differential pH measurement technology avoids issues such as poisoning and issues caused by group loop current.
Manufacturers can meet their goals of improved operation efficiency, reduced energy costs, and fewer incidents by accurately measuring and monitoring pH in brine solutions.
What Next
Every Tuesday over the next 5 weeks I take a deeper look into “Three Ways to Improve pH Measurements in Brine.”
If you can’t wait until next week or your process is not within brine and are having difficulties with online pH measurements, download the Yokogawa pH sensor lifetime ebook for reference tips to look at.




